By Sarah M. Wiles
The U.S. Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense has a robust development process for new CBRN medical countermeasures. Sarah M. Wiles explains the process and its rationale.
The strategic development of medical countermeasures (MCMs) to build biodefense resilience for the Joint Force is a top priority within the Joint Project Manager for Chemical, Biological, Radiological, and Nuclear Medical (JPM CBRN Medical).
A component of the Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND), the JPM CBRN Medical is dedicated to delivering quality, safe, and effective integrated medical solutions to counter CBRN threats to the Joint Force. Without a primary understanding of the Department of Defense Adaptive Acquisition Framework, significant planning, collaboration, resources, and agility across a multitude of government and non-government representatives and departments, this organization would be unable to effectively take a directive from conception through industrialization and ultimately place it into the hands of warfighters around the world.
MCMs such as biologics, drugs, and devices are critical for minimizing morbidity and mortality of not only warfighters but also of the general public in the event of large-scale health emergencies. Taking the directives from the Joint Requirements Office (JRO) to develop and deliver a pharmaceutical or device from paper to product-in-hand is a complex process, as is the acquisition processes behind it.
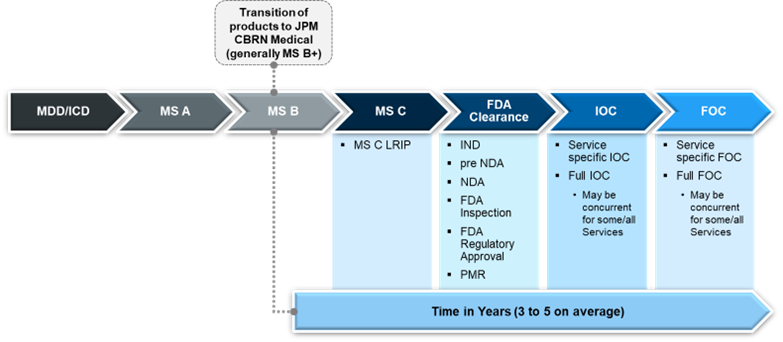
The purpose of pharmaceutical and device capability development is to assess, prevent, mitigate, reverse, and defeat the impact of CBRN threats, and provides support across a range of environments, from peacekeeping to contested environments and beyond. In most processes, the initial step is often the development of an initial capabilities document, borne from a requirement prioritized by the JRO. The initial capabilities document then moves the concept forward to materiel development decisions, which are the mandatory entry point for any major capability acquisition process. These decisions then allow for solution analysis – the process of studying and understanding a problem or system to find a solution – and begin to move that capability through a chain of events that will facilitate maturation of the product.

Major Milestones in the Acquisition Lifecycle
There are three major milestones, or checkpoints, involved in the acquisition lifecycle: technology maturation and risk reduction, engineering and manufacturing development, and production and deployment. Each of these milestones verify the of feasibility of effectively and reasonably producing the technology. When a new program concept reaches the first milestone, the goal is to determine if a program has met all existing requirements of the materiel solution analysis phase to proceed with prototyping and risk reduction.
The second milestone of engineering and manufacturing development is often considered to be a relatively “big” milestone as it is here that the future of the program and the development of the MCM is often assured, receiving formal cost-schedule-technical baselines.
Finally, during production and deployment, the process really gains traction as the technology is placed in the hands of potential users, ultimately assisting in final development requirements that will ensure the success of the product.
Once a technology arrives at production and deployment, true industrialization is just beginning. An initial small run, called low-rate initial production, is completed and deployed for initial operational test and evaluation. This is the final evaluation involving dedicated operational testing that answers whether this product will work as intended. Articles produced during initial testing are what will build the foundation for the appropriate regulatory process. For pharmaceutical development, regulatory approval is led by the U.S. Food and Drug Administration (FDA).
Regulatory Approval of Drugs
The FDA has a well-defined process for the regulatory approval of drugs to be marketed. This begins with an investigational new drug application and is followed by a series of steps, namely clinical trials, new drug applications, regulatory approval, and finally permission to market and manufacture the drug or device. One exception to this flow pertains to diagnostic devices.
Generally, diagnostic devices undergoing production and deployment have completed a clinical study, had validation data, and adhered to FDA device regulations. Additionally, devices, while a medical product, must consider cybersecurity, hardware, and software engineering requirements and regulations. Medical devices introduce an additional level of complexity related to their review and approval process throughout the acquisition life cycle. Devices are generally cleared via a de novo or 510K regulatory pathway. De novo means it is a novel device, and 510K means there is a predicate device already approved on the market.
As the regulatory process comes to an initial conclusion and moves into the monitoring phase, the acquisition process continues concurrently with an initial operational test, evaluation, and low-rate initial production to determine whether the product will move forward into full-rate production. If so, it has a proven manufacturing process, acceptable performance reliability, and has established sustainment capability, maturing to the point of initial operational capability. This is the point where the system can meet agreed upon conditions of minimum system performance in its intended operational environment. Each service is then responsible for deployment and maintenance of the drug or device to include support, training, and logistical management processes within the operational environment.
Finally, once initial operational capability has been attained by all services, the product moves to full operational capability – marking the completion of a development effort – before finally arriving at operations and lifecycle sustainment. The work doesn’t completely end here as there are also post-market safety systems and reporting requirements imposed by the FDA to ensure continued safety and efficacy of the approved drug or device. But, in general, that concludes the process of directive to product-in-hand.
Drug Development Pathway
The JPM CBRN Medical has a successful history of MCM development, built upon the process described above. The most recent achievement is the newly developed JPM CBRN Medical Advanced Anticonvulsant System (AAS) having reached initial operational capability. This is an injection system that delivers the drug Midazolam via an autoinjector and is approved by the FDA as indicated in the treatment of status epilepticus, or sustained seizures. It can be used as one of the first-line treatments for seizures resulting from exposure to nerve agents improving upon, and eventually replacing, the currently fielded Convulsant Antidote for Nerve Agent autoinjector, which administers the drug Diazepam. Midazolam is known to terminate seizures more quickly than Diazepam, critical in cases of status epilepticus where early treatment can lead to greatly improved patient outcomes.
The pathway to initial operational capability for the AAS, with the current industry partner, began in August 2020 with the signing and implementation of an Other Transaction Authority agreement to collaborate with a public partner. The program, working with its present manufacturing partner, employed all available resources to expedite development. When the AAS moved into the regulatory approval process with the FDA, our organization utilized Public Law 115-92, the Federal Food, Drug, and Cosmetic Act amendment that “authorizes additional emergency uses for medical products to reduce deaths and severity of injuries caused by agents of war” as a foundation for enhanced communication and collaboration between our agreement partner, the JPM CBRN Medical, and the FDA. The AAS autoinjector moved from new drug application submission to regulatory approval in only five months, highlighting the value of Public Law 115-92 to the Joint Force. The AAS autoinjector reached initial operational capability in November of 2023, with full operational capability planned for September 2025.
Next Generation Diagnostics System 1
Another success story within the JPM CBRN Medical is the Next Generation Diagnostics System 1 (NGDS 1) – a diagnostic device designed to protect the Joint Force through the identification of biological hazards. The NGDS 1 is a fully automatic diagnostic platform that can detect a variety of viruses, bacteria, toxins, and training targets within its two panels. These are “Warrior”, an FDA-approved medical diagnostic panel, and “Sentinel”, an environmental detection panel. The development and success of the NGDS 1 is yet another example of how the JPM CBRN Medical was able to utilize the acquisition process and deliver vital diagnostic capabilities to the warfighter. The NGDS 1 effort began with a materiel development decision in October 2010 to respond to the need for reliable identification and diagnosis of bacteria, viruses, and toxins post-exposure. The system then moved through the acquisition process before reaching full operational capability across the U.S. in August 2020.
NGDS 1 performance has been so successful that it is now globally positioned and supports national preparedness in both public health and biodefense settings. However, the JPM CBRN Medical is not stopping there. NGDS development is evolving, with current efforts focused on the NGDS 2 – Chemical Diagnostics, or “ChemDX”. This is a separate, rapid, handheld, point-of-care device that will provide quantitative detection of acetylcholinesterase levels in persons with suspected exposure to nerve agents, for example.
This capability, as with that of the original NGDS 1 system, will assist providers in informed medical treatment and plans of care, in addition to providing supportive evidence for command decision making. The NGDS 2 – Man Portable Diagnostic System brings a system using the polymerase chain reaction detection technology employed in NGDS 1 to more austere, deployed medical settings.

Conclusion
By linking top technical and scientific expertise, through adaptive acquisition mechanisms, to biodefense requirements, the identification and advanced development of MCMs enables risk awareness and detection, informs decision-making, allows rapid response to limit bio-incident impacts, increases biodefense enterprise preparedness, and facilitates recovery after an incident.
Additionally, these efforts support the intent of the 2023 Biodefense Posture Review, initiated by the National Biodefense Strategy, which underpins the need to manage risk, improve preparedness, and provide a rapid, resilient, and balanced response to biological threats. Synchronizing with such strategy, our organization has the proven ability to provide the Joint Force with the medical tools and defenses they require. In other words, this work furthers the comprehensive capability of the CBRN MCM portfolio and shows tangible progress on biodefense objectives.

Sarah M. Wiles is a business analyst with the Joint Project Manager for Chemical, Biological, Radiological, and Nuclear Medical’s Strategy and Integration Office. Wiles holds a B.S.N. from Frostburg State University, an M.S. in Informatics from the University of South Florida, and a Graduate Certificate in Agile Project Management from Georgetown University. She can be reached at [email protected].





